Medical Device Testing and Certification Market Expected to Hit USD 10.2 Billion by 2035 with a Remarkable 6.37% CAGR
The market is segmented by service type into testing services and certification services.
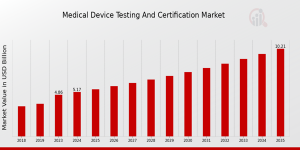
US, NY, UNITED STATES, August 6, 2025 /EINPresswire.com/ -- Medical Device Testing and Certification Market Expands with Surge in Regulatory Demand and InnovationThe Medical Device Testing and Certification Market was valued at USD 4.86 billion in 2023, reflecting growing regulatory emphasis on safety, quality, and performance across global healthcare systems. According to MRFR analysis, the market is set to climb to USD 5.17 billion in 2024, with projections reaching approximately USD 10.2 billion by 2035. This expansion corresponds to a compound annual growth rate (CAGR) of 6.37% from 2025 to 2035. Factors driving this growth include increasingly stringent compliance standards, the rapid pace of medical device innovation, and rising international demand for third-party certification to ensure global market access.
Market Scope and Industry Size
As healthcare devices become increasingly digital, portable, and connected, ensuring their safety, accuracy, and compliance has become a top priority. The growing industry size is fueled by strong demand across developed and emerging markets alike. With rising regulatory scrutiny and the globalization of healthcare production and distribution, device testing and certification services are now an indispensable part of the medical device lifecycle.
The market’s scope includes all forms of regulatory, quality, and performance testing for active, non-active, implantable, and in vitro diagnostic (IVD) devices. Additionally, regulatory bodies across North America, Europe, and Asia-Pacific are mandating strict pre- and post-market evaluations, further accelerating service demand.
Segment Growth and Market Share
The market is segmented by service type into testing services and certification services. Testing services account for the largest market share, owing to their critical role in safety, electrical, mechanical, and biological compatibility assessments. This segment continues to grow as medical device designs become more intricate and software-driven.
Certification services—including CE marking, ISO 13485 certification, and FDA approvals—are also witnessing strong segment growth, especially as manufacturers seek global market access. Increasing exports and regional harmonization efforts are prompting companies to invest more in high-quality certification to reduce time to market.
By sourcing, the outsourced segment holds a dominant position. Manufacturers are increasingly relying on third-party laboratories and certification bodies to streamline processes, access specialized expertise, and meet fast-evolving international standards efficiently.
In terms of device type, active implantable devices and IVD medical devices are at the forefront. Both categories require intensive testing due to their high risk and regulatory classification, thus representing a significant portion of global testing demand.
Top Companies Driving the Market
Several top companies lead the global medical device testing and certification space, including:
Deloitte
Intertek
BSI Group
Bureau Veritas
DEKRA
UL
SGS
Eurofins Scientific
NSF International
Applus+
These firms offer full-spectrum testing and certification services and have continued to expand their capabilities through digital transformation, laboratory network expansion, and strategic partnerships. Their ability to offer region-specific as well as global compliance solutions gives them a competitive edge.
Emerging Trends and Innovation
One of the most significant emerging trends is the integration of digital technologies such as AI, machine learning, and IoT simulations into testing environments. These technologies are enabling faster and more reliable testing outcomes, particularly for software-based and connected medical devices.
In addition, remote auditing and virtual inspections have become standard practice in post-pandemic workflows. These innovations support global supply chain continuity while enabling regulatory compliance even under mobility restrictions.
Opportunities in the Global Market
Emerging markets, particularly in the Asia-Pacific region, are providing ample opportunities due to rising healthcare investments, manufacturing expansion, and favorable regulatory reforms. Countries such as India, China, and South Korea are witnessing an influx of medical device exports and startups, intensifying the need for third-party testing partners.
The increasing focus on home healthcare, wearable medical devices, and personalized diagnostics is also creating new demand for specialized testing protocols, opening growth opportunities for niche testing services and software verification labs.
Recent Developments and Strategic Moves
The market has seen several recent developments, including expansions of digital testing infrastructure and collaborations between certification agencies and national regulatory authorities. Firms like TÜV SÜD and Eurofins have broadened their lab capabilities to support faster testing for connected devices and cybersecurity evaluations.
Additionally, many companies are embracing AI-driven quality assurance tools, allowing them to optimize test cycles and deliver results with higher accuracy and shorter turnaround times. These developments are improving operational efficiency while helping clients meet international regulatory deadlines.
Future Outlook for Stakeholders
Looking ahead, the future outlook for the medical device testing and certification market is highly positive. As the medical device industry continues to innovate—especially in AI-enabled, wearable, and implantable technologies—the need for robust, third-party validation will only intensify.
Healthcare systems, regulatory bodies, and medical technology developers must align strategically to meet the dual goals of patient safety and market competitiveness. The evolution of cybersecurity, interoperability standards, and software-as-a-medical-device (SaMD) requirements will drive further market expansion.
Related Report:
Neuropathic Pain Market : https://www.marketresearchfuture.com/reports/neuropathic-pain-market-1390
Radiology Information System Market : https://www.marketresearchfuture.com/reports/radiology-information-system-market-2464
Thyroid Disorder Market : https://www.marketresearchfuture.com/reports/thyroid-disorder-market-2747
Ambulatory Services Market : https://www.marketresearchfuture.com/reports/ambulatory-services-market-2491
Micro pump Market : https://www.marketresearchfuture.com/reports/micro-pump-market-1300
Artificial Intelligence in Healthcare Market : https://www.marketresearchfuture.com/reports/healthcare-artificial-intelligence-market-5681
Concussions Market : https://www.marketresearchfuture.com/reports/concussions-market-5724
Renal Disease Market : https://www.marketresearchfuture.com/reports/renal-disease-market-3167
Actinic Keratosis Treatment Market : https://www.marketresearchfuture.com/reports/actinic-keratosis-treatment-market-2366
Myelodysplastic Syndrome Drugs Market : https://www.marketresearchfuture.com/reports/myelodysplastic-syndrome-drugs-market-8134
About Market Research Future:
Market Research Future (MRFR) is a global market research company that takes pride in its services, offering a complete and accurate analysis with regard to diverse markets and consumers worldwide. Market Research Future has the distinguished objective of providing the optimal quality research and granular research to clients. Our market research studies by products, services, technologies, applications, end users, and market players for global, regional, and country level market segments, enable our clients to see more, know more, and do more, which help answer your most important questions.
Sagar Kadam
Market Research Future
+1 628-258-0071
email us here
Visit us on social media:
LinkedIn
Facebook
X
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.